Abstract
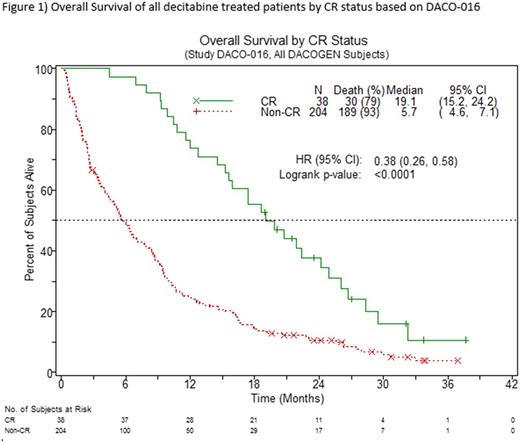
The correlation between complete remission (CR) and overall survival (OS) is not well defined for patients with AML who are not eligible for intensive chemotherapy (ICT). With the introduction of hypomethylating agents (HMAs), outcomes have improved compared to low-dose cytarabine or best supportive care, however, there is a need to understand whether patients who achieve a CR following treatment with HMAs experience a longer OS compared to those patients who don't achieve a CR. This retrospective analysis of CR and OS is based on data from two clinical trials of decitabine, DACO-0161 and DACO-0172. The dose schedule of decitabine in both studies was 20 mg/m2 intravenously for 5 consecutive days of continuous 4-week cycles. In DACO-016, the control arm was Treatment Choice (TC; supportive care or cytarabine 20 mg/m2 per day as a subcutaneous injection for 10 consecutive days every 4 weeks). DACO-017 was a single arm study. Morphologic CR was based on the modified 2003 International Working Group criteria. OS was measured from the date of randomization (N=485) in DACO-016 and the date of starting decitabine treatment (N=55) in DACO-017, with patients last known to be alive censored at the last date of contact. OS stratified by CR was estimated using the Kaplan-Meier method and tested using the log-rank test. In DACO-016, 15.7% (N=38) of patients treated with decitabine achieved CR. The median OS for decitabine treated patients who achieved a CR was 19.1 months (95% confidence interval (CI): 15.2-24.2). 97.4% of CRs (N=37) occurred at or after the fourth cycle of decitabine. The median OS for patients who did not achieve a CR with decitabine was 5.7 months (CI: 4.6-7.1) (Figure 1). In contrast, only 7.4% (N=18) of patients treated with TC achieved a CR. In the TC arm, the median OS for patients who achieved a CR was 25.0 months (CI: 16.3-37.0) and 4.5 months (CI: 3.8-5.5) for patients who did not achieve a CR. In DACO-017, the median OS for patients who achieved a CR was 21.6 months (CI: 7.1-24.7). For patients who did not achieve a CR, the median OS was 6.1 months (CI: 3.1-8.1). The median time to CR for decitabine treated patients was 4.6 months. Both studies showed that the median OS of patients who achieved a CR was more than twice that of patients who did not achieve a CR. This analysis demonstrates the prognostic value of CR in relation to OS in patients with AML regardless of treatment and thus the importance of treating patients with decitabine for a minimum of 4 cycles to before evaluating response to treatment. While CR has been shown to be an imperfect surrogate for OS, in this analysis of patients with AML who were ineligible for ICT, achieving a CR remains associated with improved OS benefits vs. not achieving a CR.
References:
1. Kantarjian HM , Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R, Cermak J, Kuo CY, Oriol A, Ravandi F, Faderl S, Delaunay J, Lysák D, Minden M, Arthur C., Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012 Jul 20;30(21):2670-7. doi: 10.1200/JCO.2011.38.9429. Epub 2012 Jun 11.
2. Cashen AF, Schiller GJ, O'Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010 Feb 1;28(4):556-61. doi: 10.1200/JCO.2009.23.9178. Epub 2009 Dec 21.
He: Janssen: Employment. Doyle: Janssen: Employment. Xiu: Janssen: Employment. Nemat: Johnson & Johnson, LLC: Equity Ownership; Janssen: Employment. Loefgren: Janssen: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal